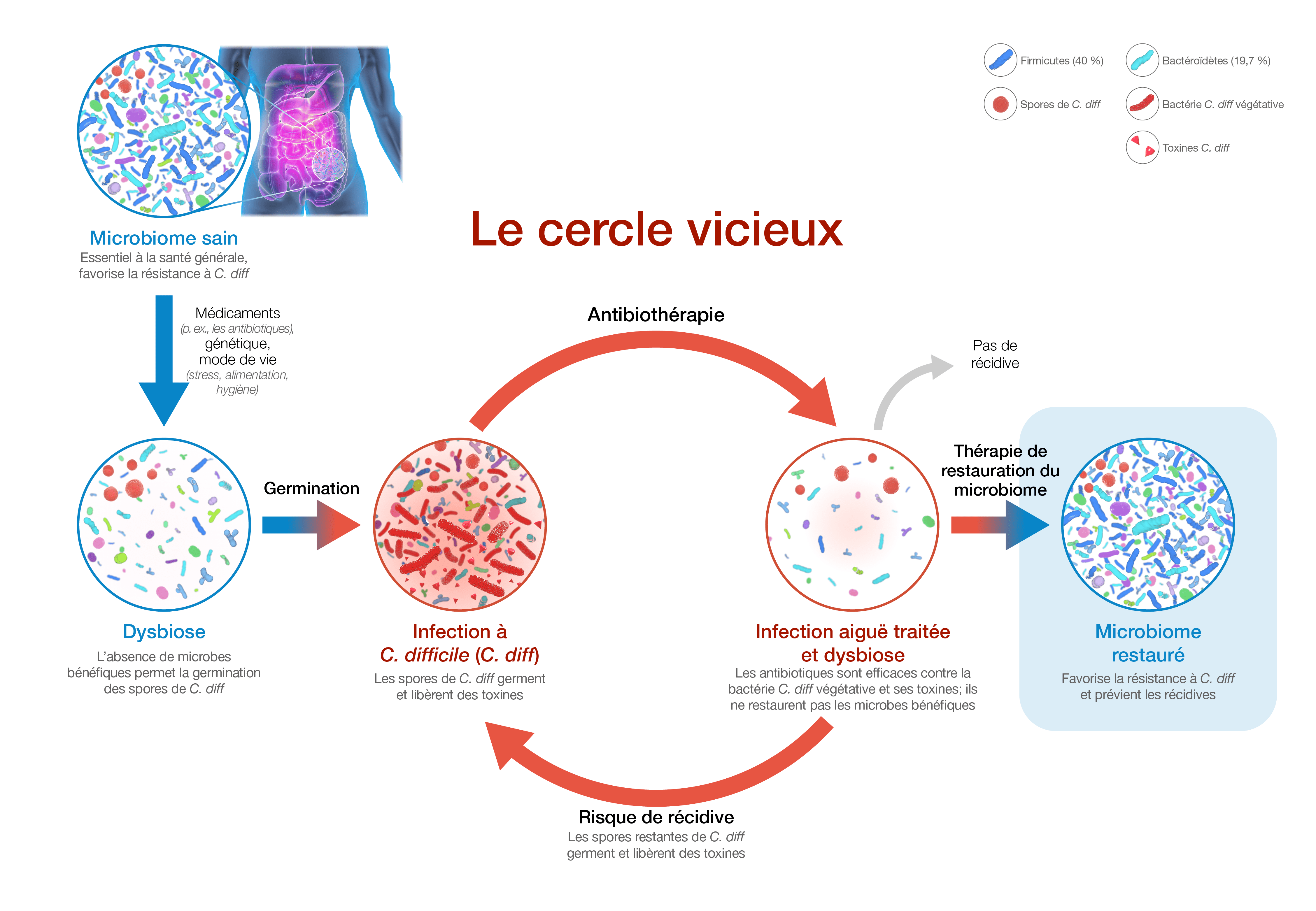
-
Langdon A, Schwartz DJ, Bulow C, et al. Microbiota restoration reduces antibiotic-resistant bacteria gut colonization in patients with recurrent Clostrididoides difficile infection from the open-label PUNCH CD Study. Genome Med. 2021;13:28.
-
Seekatz AM, Safdar N, Khanna S. The role of the gut microbiome in colonization resistance and recurrent Clostridioides difficile infection. Therap Adv Gastroenterol. 2022;15:1-18.
-
Langdon A, Crook N, Dantas G. The effects of antibiotics on the microbiome throughout development and alternative approaches for therapeutic modulation. Genome Med. 2016;8(1):39.
-
Normington C, Chilton CH, Buckley AM. Clostridioides difficile infection; new treatments and future perspectives. Curr Opin Gastroenterol. 2024;40(1):7-13.
-
Khanna S. Microbiota restoration for recurrent Clostridioides difficile: Getting one step closer every day! J Intern Med. 2021;290(2):294-309.
-
Schwartz DJ, Langdon AE, Dantas G. Understanding the impact of antibiotic perturbation on the human microbiome. Genome Med. 2020;12(82):1-12.
-
Ashraf MF, Tageldin O, Nassar Y, et al. Fecal Microbiota Transplantation in Patients With Recurrent Clostridium difficile infection: A Four-Year Single Center Retrospective Review. Gastroenterology Res. 2021;14(4):237-243.
-
Van Nood E, Vrieze A, Nieuwdorp M, et al. Duodenal infusion of donor feces for recurrent Clostridium difficile. N Engl J Med. 2013;368(5):407-415.
-
Quay T, Walter M. Fecal Microbiota Therapy in Canada: An Environmental Scan. CADTH. 2020;(95):1-36.
-
Gawey BJ, Khanna S. Clostridioides difficile Infection: Landscape and Microbiome Therapeutics. Gastroenterol Hepatol (N Y). 2023;19(6):319-328.
-
U.S. Food and Drug Administration website. Fecal Microbiota for Transplantation: Safety Alert–Risk of Serious Adverse Events Likely Due to Transmission of Pathogenic Organisms. Accessed March 12, 2022, at https://www.fda.gov/safety/medical-product-safety-information/fecal-microbiota-transplantation-safety-alert-risk-serious-adverse-events-likely-due-transmission.
-
Tariq R, Pardi DS, Bartlett MG, et al. Low cure rates in controlled trials of fecal microbiota transplantation for recurrent Clostridium difficile infection: a systematic review and meta-analysis. Clin Infect Dis. 2019;68(8):1351-1358.
-
Cordaillat-Simmons M, Rouanet A, Pot B. Live biotherapeutic products: the importance of a defined regulatory framework. Exp Mol Med. 2020;52(9):1397-1406.
-
Kelly CR, Fischer M, Allegretti JR, et al. ACG Clinical Guidelines: prevention, diagnosis, and treatment of Clostridioides difficile infections. Am J Gastroenterol. 2021;116(6):1124-1147.
-
Suez J, Zmora N, Ziblerman-Schapira G, et al. Post-antibiotic gut mucosal microbiome reconstitution is impaired by probiotics and improved by autologous FMT. Cell. 2018;174(6):1406-1423.e16.